Practical Overview of Multiplex Immunohistochemistry using TSA
Simultaneous detection of multiple distinct proteins of interest within a single sample can be achieved with carefully optimized fluorescent multiplex immunohistochemistry (mIHC) using tyramide signal amplification (TSA). This technique utilizes an unconjugated primary antibody specific to the protein of interest, and a primary specific secondary antibody conjugated to horseradish peroxidase (HRP). Detection is achieved with the fluorophore-conjugated HRP substrate, tyramide. When activated, tyramide forms covalent bonds with the tyrosine residues on or near the protein of interest. This permanent deposition allows the primary/secondary antibody pair to be stripped from the sample without disrupting the antigen-associated fluorescence signal. Thus, multiple rounds of staining can be performed in sequence on a given sample, without fear of the crosstalk that would otherwise result from using multiple primary antibodies raised in a single species.
The basic procedure for fluorescent mIHC with TSA is as follows:
- Slide-mounted formalin-fixed paraffin embedded tissue sections are deparaffinized in Xylene and hydrated in successive treatments with ethanol and distilled water.
- Antigen retrieval, in which the masking of an epitope is reversed, is needed to facilitate epitope-antibody binding, which is accomplished by heat-induced epitope retrieval (HIER). HIER can be performed by heating the slides immersed in retrieval buffer with a steamer, microwave, or other dedicated HIER instruments providing the heat source. The most commonly used HIER buffers are a citrate pH 6.0 or Tris-EDTA pH 9.0. The choice of buffer will depend on the antibody manufacturer’s specifications.
- After antigen unmasking, sections are incubated with an IHC-validated primary antibody. The optimal concentration of the primary antibody must be determined empirically to ensure a high signal to noise ratio; that is, a strong fluorescence signal intensity and minimal background for each target of interest.
- Following incubation with primary antibody, sections are washed and incubated with the HRP-conjugated secondary antibody, which is specific to the primary antibody. The HRP enzyme is needed to catalyze the tyramide reaction which leads to fluorophore being covalently deposited onto the tissue. This means the fluorophore will remain on the tissue even if the antibodies are removed.
- After washing the slides, fluorophore-conjugated tyramide is applied. The choice of fluorophore should be optimized to the primary antibody, taking expression level of the target protein and fluorophore intensity into account. In general, an antibody against a low abundance target should be paired with a high intensity fluorophore. Conversely, an antibody against a high abundance target should be paired with a relatively low intensity fluorophore. This is done in an effort to balance signal intensities of the multiple proteins of interest.
- Steps two through five are essentially a staining cycle which is repeated for each target of interest. Subsequent HIER steps serve to strip primary/secondary antibodies from sample and to unmask the epitope for the next primary. Each cycle of staining uses a distinct tyramide-fluorphore conjugate. The order in which primary antibodies are applied in the multiplex panel must be carefully designed, however, since signal loss and epitope damage increase with each successive cycle of staining. Once all targets have been probed, the slides can be counterstained with DAPI, mounted, and imaged using an appropriate imaging platform.
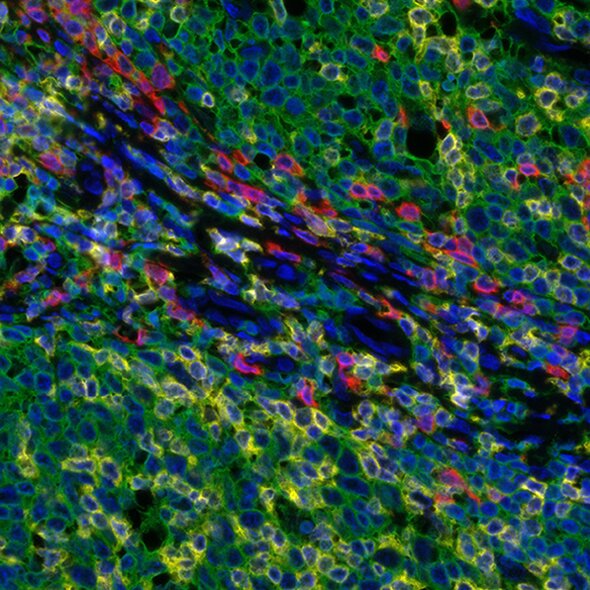
Detection of human CD3 (yellow), CD8 (red), and CD20 (green) in FFPE tonsil by IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12] (A700-016), rabbit anti-CD8a recombinant monoclonal [BLR044F] (A700-044), mouse anti-CD20 monoclonal [L26] (A500-017A). Secondary: HRP-conjugated goat antirabbit IgG (A120-501P) and HRP-conjugated goat anti-mouse IgG (A90-116P). Substrate: Opal™ 520, 620, and 690. Counterstain: DAPI (blue).
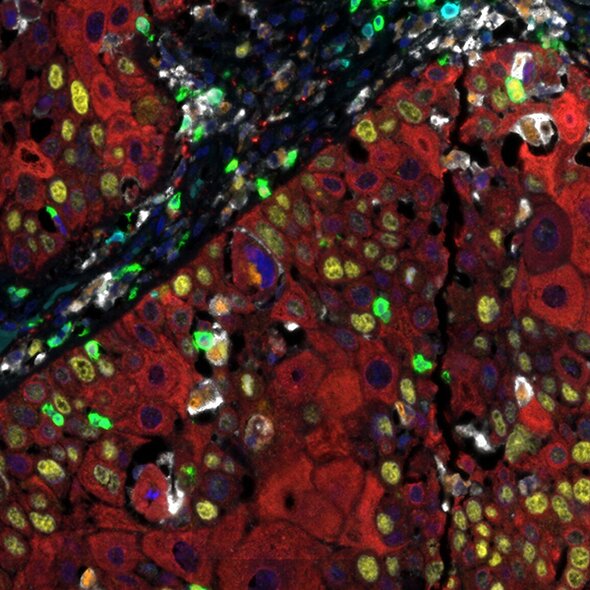
Detection of human CD3 (teal), CD8 (green), CD68 (orange), CK (red), Ki67 (yellow) and PD-L1 (white) in FFPE HNSCC by IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12] (A700-016), rabbit anti-CD8a recombinant monoclonal [BLR044F] (A700-044), mouse anti-CD68 monoclonal [KP-1] (A500-018A), mouse anti-cytokeratin monoclonal [AE1/AE3] (A500-019A), rabbit anti-Ki67 monoclonal [BLR021E] (A700-021) and rabbit anti-PD-L1 recombinant monoclonal [BLR020E] (A700-020). Secondary: HRP-conjugated goat anti-rabbit IgG (A120-501P) and HRP-conjugated goat anti-mouse IgG (A90-116P). Substrate: Opal™ 480, 520, 570, 620, 690 and 780. Counterstain: DAPI (blue).
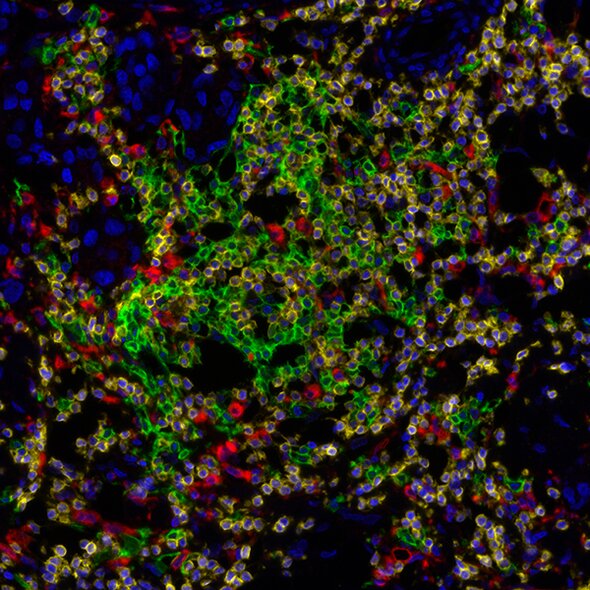
Detection of human CD3 (yellow), CD20 (green), and CD68 (red) in FFPE lung carcinoma by IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12] (A700-016), mouse anti-CD20 monoclonal [L26] (A500-017A), and mouse anti-CD68 monoclonal [KP-1] (A500-018A). Secondary: HRP-conjugated goat antirabbit IgG (A120-501P) and HRP-conjugated goat anti-mouse IgG (A90-116P). Substrate: Opal™ 520, 620, and 690. Counterstain: DAPI (blue).