Direct vs. Indirect Staining for Flow Cytometry
Flow cytometry is a high-throughput technique that analyzes cell populations at the single cell level. This assay relies on detecting fluorescence to identify specific intracellular and extracellular targets, which can be done through direct and indirect staining. Direct staining uses primary antibodies recognizing your target of interest that are conjugated to fluorophores. Indirect staining uses unlabeled primary antibodies that are subsequently detected by fluorophore-labeled secondary antibodies recognizing the species and isotype of the primary antibody. Both methods have advantages and limitations where understanding the details of your experiment will help you select the best protocol for your situation.
Direct Staining
Direct staining is a one-step procedure where fluorophore-conjugated primary antibodies are used to detect the targets of interest in your flow cytometry experiment. This single-step detection method is quick and easy to perform. The use of conjugated primary antibodies may make multiplexing easier because the antibodies used may be produced from the same host species. Since secondary antibodies are not used, background fluorescence in your sample is reduced. However, this also means that there is no signal amplification, which does occur when secondary antibodies are used. Additionally, there is frequently an increased cost associated with directly conjugated primary antibodies as compared to unconjugated primary antibodies. Using directly conjugated antibodies also locks you into a specific fluorescence parameter for that primary antibody once your flow cytometry protocol is optimized.
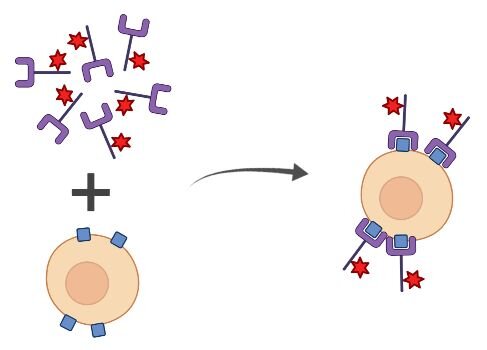
Pros
- One-step protocol for target detection, making flow cytometry staining quick and easy
- Better for multiplexing – can use primary antibodies from the same species
- No background from secondary antibodies
Cons
- No signal amplification from secondary antibodies possible
- Conjugated primary antibodies may be expensive
- Locked into a specific fluorescence channel for each target antigen (cannot change between fluorophores without optimizing your panel again)
Indirect Staining
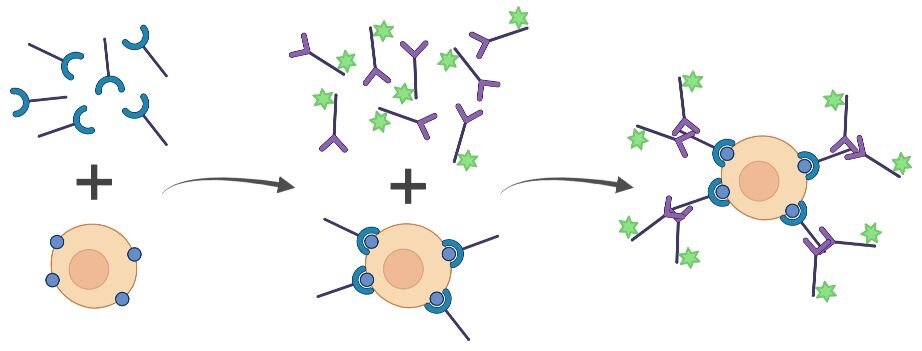
Indirect staining uses an unconjugated primary antibody followed by a fluorophore-conjugated secondary antibody for flow cytometry that detects the primary antibody. This process requires sequential staining steps, making it a longer protocol than direct staining. One of the most significant benefits of indirect staining is that multiple secondary antibody molecules bind to each primary antibody, amplifying low-abundance targets. Therefore, indirect staining has increased sensitivity compared to direct staining. Additionally, the ability to interchange primary and secondary antibody combinations makes indirect staining more flexible and cost-effective. The addition of the secondary antibody may increase the background fluorescence in the sample. However, including the appropriate secondary-only controls can account for this possibility. Indirect staining may also make the development of multiplex panels more challenging because primary antibodies from different host species are required so that each target has a unique primary-secondary pair.
Pros
- Increased sensitivity – multiple secondaries can detect a single primary antibody – amplifies lowly expressed targets
- Use of a single secondary antibody with multiple primary antibodies may increase flexibility and save money
Cons
- May have increased background
- Challenges with multiplexing – finding good combinations of primary and secondary antibodies
Indirect and Direct Staining
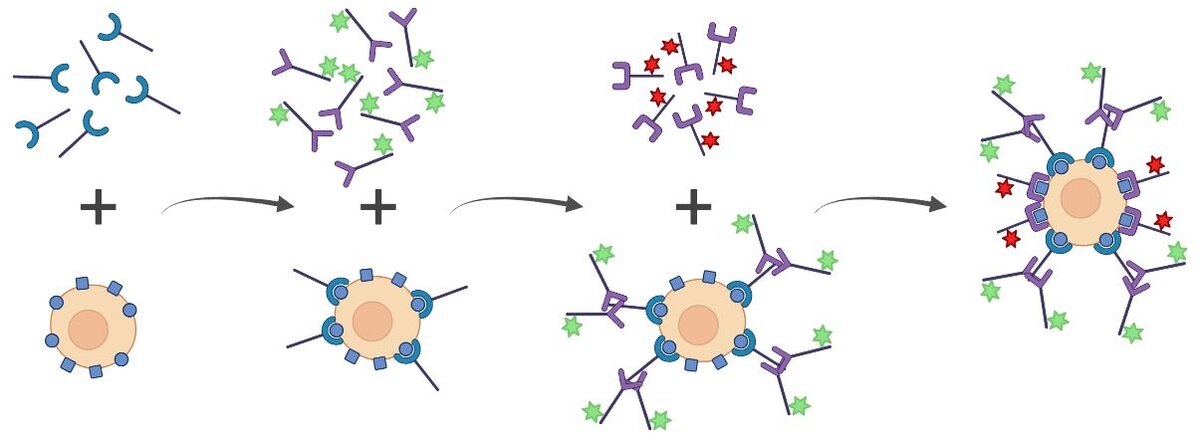
Indirect and direct staining methods may be combined to increase flexibility and adaptability of single cell analysis by flow cytometry. One benefit of combining the two staining methods is that indirect staining may be used on the lower expressed targets to amplify their signal.
The combination of the two staining methods into a single protocol creates a sequential staining process. While this method increases the flexibility of the staining process, careful planning is necessary to ensure that the secondary antibodies do not amplify/detect the conjugated antibodies resulting in potential false double-positive populations. If antibodies from the same host species are being used for both the directly conjugated primary antibodies and the unconjugated antibodies, this will add an additional step to the staining protocol.
Key Takeaways
| Direct Staining | Indirect Staining | |
|---|---|---|
| Description of Method | Primary antibodies are directly conjugated to a fluorophore. Combining the antibody and cell sample results in a detectable signal. | Primary antibodies are unconjugated, meaning they are not bound to a fluorophore. Thus, a secondary antibody conjugated to a fluorophore must be used to detect the signal. |
| Advantages |
|
|
| Disadvantages |
|
|
Successful staining for flow cytometry, whether using the direct or indirect method, requires optimization of your protocols, titration of antibodies, and use of appropriate controls. Understanding the limitations of each method along with the antibodies available for your targets will help you to create the optimal experimental design for your specific circumstances. Happy planning!