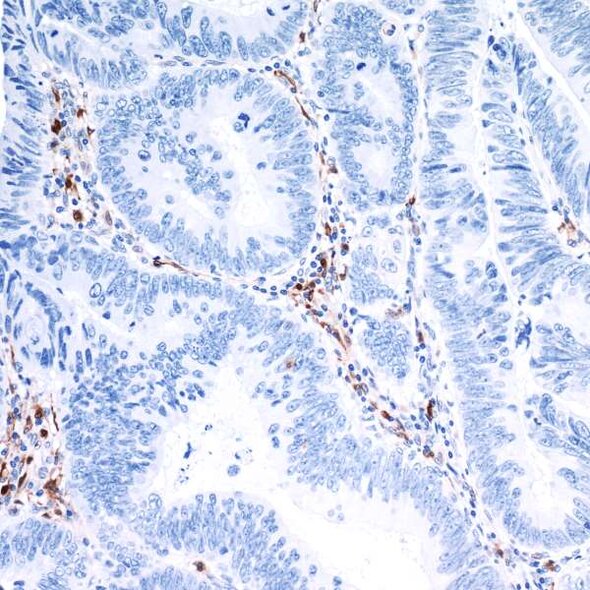
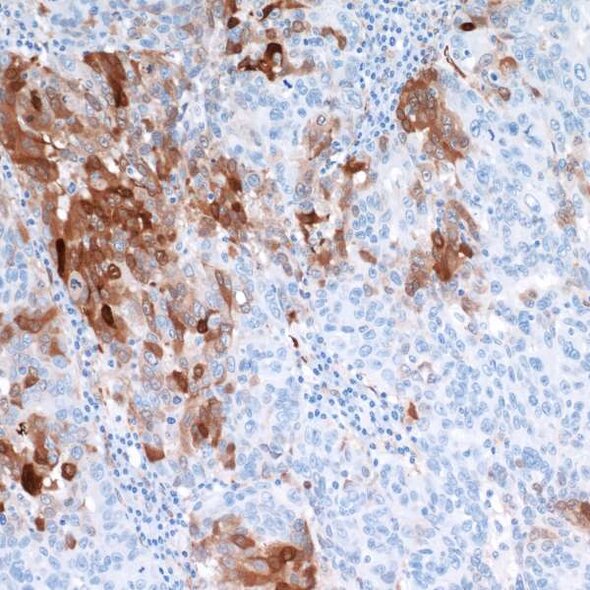
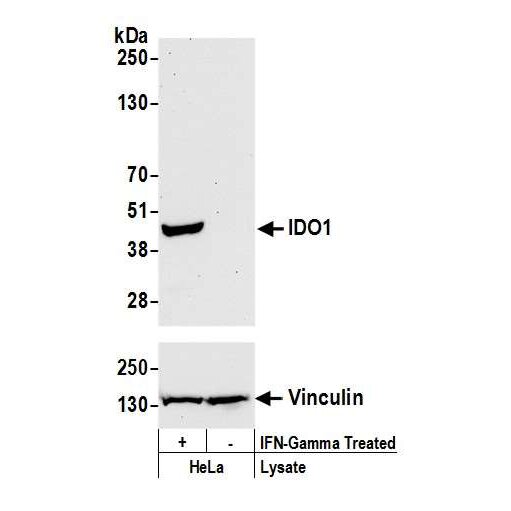
IDO1 - An intracellular target for cancer therapy
Indoleamine 2,3-dioxigenase 1 (IDO1) is an enzyme that catalyzes the rate limiting step in the conversion of the essential amino acid tryptophan to kynurenine. IDO1 was first identified in the 1950s, but it wasn’t until the 1990s that the immunosuppressive nature of the enzyme was described. IDO1 is expressed in both primary and metastatic tumor tissue, and its expression is correlated with advanced disease stage and decreased survival.3,4 Both substrate (tryptophan) and product (kynurenine) of the IDO1-catalyzed reaction can be measured in blood, and an increased ratio of product to substrate has been associated with poor prognosis in breast, cervical, and non-small cell lung cancer, as well as in acute myeloid leukemia and melanoma.4
The mechanism by which IDO1 activity is associated with poor prognosis in cancer may be explained in that T cell function can be suppressed by both tryptophan depletion and by the presence of kynurenine.4 Tryptophan depletion can lead to accumulation of uncharged tryptophan transfer RNA (tRNA). Uncharged tRNA is sensed by the stress response kinase general control nonderepressible 2 (GCN2), which impairs T cell activation in microenvironments with high IDO1 activity.5 In addition to suppression of T cell activation though tryptophan depletion, kynurenine is a key signaling molecule that promotes transcription of immunosuppressive mediators and development of regulatory T cell populations.6
Together, these effects allow cells that overexpress IDO1 to evade immune surveillance. Consequently, developing therapies that target the IDO1 pathway may provide novel anti-cancer treatment options since a significant portion of cancer patients do not respond to traditional therapies.6 Combining an IDO1 inhibitor with a second treatment modality may expand the responsive patient pool since IDO1 inhibitors can enhance the efficacy of other cancer treatments such as chemotherapy, radiotherapy, and immune checkpoint therapy without increasing their side effects.7,8
Unlike cell-surface checkpoint receptor molecules that can be targeted by antibody-based therapeutics, IDO1 and its downstream effectors are intracellular. Additionally, IDO1 is a single chain catalytic enzyme with a well-defined biochemical action and is one of only a few enzymes which catabolize tryptophan.6 Together, these properties make IDO1 a good target for small molecule inhibitors.
The first generation small molecule IDO1 inhibitors have failed to demonstrate significant anti-tumor activity when provided as a monotherapy in patients with advanced cancers.9 However, more significant response has been observed when IDO1 inhibitors have been combined with an immune checkpoint inhibitor, with the most profound responses seen in renal cell carcinoma, squamous cell cancer of the head and neck, non-small cell lung cancer, and melanoma.6 Multiple distinct small molecule IDO1 inhibitors are currently in clinical development.
Bethyl offers IDO1 Recombinant Monoclonal Antibodies from BLR031F and BLR040F.
References
- Boyland E, Williams DC. 1956. The metabolism of tryptophan. 2. The metabolism of tryptophan in patients suffering from cancer of the bladder. Biochem J. Nov;64(3):578-582.
- Munn DH, Shafizadeh E, Attwood JT, et al. 1999. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. May;189(9):1363-1372.
- Godin-Ethier J, Hanafi LA, Piccirillo CA, et al. 2011. Indoleamine 2,3-dioxygenase expression in human cancers: clinical and immunologic perspectives. Clin Cancer Res. Nov;17(22):6985-6991.
- Brochez L, Chevolet I, Kruse V. 2017. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer. May;76:167-182.
- Mellor AL, Munn DH. 1999. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. Oct;20(10):469-473.
- Cheong JE, Sun L. 2018. Targeting the IDO1/TDO2-KYN-AhR pathway for cancer immunotherapy - challenges and opportunities. Trends Pharmacol Sci. Mar;39(3):307-325.
- Muller AJ, DuHadaway JB, Donover PS, et al. 2005. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. Mar;11(3):312-319.
- Monjazeb AM, Kent MS, Grossenbacher SK, et al. 2016. Blocking indolamine-2,3-dioxygenase rebound immune suppression boosts antitumor effects of radio-immunotherapy in murine models and spontaneous canine malignancies. Clin Cancer Res. Sep;22(17):4328-4340.
- Prendergast GC, Malachowski WP, DuHadaway JB, et al. 2017. Discovery of IDO1 inhibitors: from bench to bedside. Cancer Res. Dec;77(24):6795-6811.