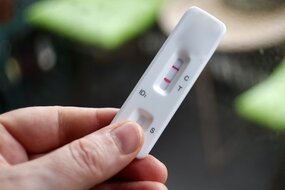
LFA Antibodies & Antigens
Fortis Life Sciences® provides high-quality antibodies and antigens for lateral flow assay (LFA) and rapid diagnostic test development. Our LFA-validated reagents enable manufacturers to create high-performance lateral flow immunoassays for point-of-care testing across applications, from drugs-of-abuse screening to infectious disease detection, ideal for at-home self-testing, disease-screening, and clinical diagnostics.
Under the Arista Biologicals® brand, we develop monoclonal antibodies, polyclonal antibodies, and purified antigens optimized for LFA manufacturing, including matched-pair reagents for diverse rapid test formats, with bulk pricing options and reliable supply.