Spatial Biology
Spatial biology reveals the intricate interactions between cells and within the tissue microenvironment, which includes the extracellular matrix, immune cells, stromal cells, and the vasculature.
Multiplex Immunofluorescence
Multiplex immunofluorescence is a powerful tool within the field of spatial biology, as it allows researchers to simultaneously visualize and analyze the expression patterns and spatial distribution of numerous proteins within a tissue sample, providing valuable insights into cellular interactions, tissue architecture, and disease processes.
Multiplexing techniques use several antibodies and fluorescent detection to target specific proteins within the tissue microenvironment, allowing researchers to create a detailed map of the various cells and proteins and their interactions. In clinical settings, spatial information can be used to determine the best therapy for a particular patient. At scale, it can also assist in the identification of novel biomarkers and the development of new therapeutics based on key cell-cell interactions.
Tyramide Signal Amplification
Tyramide signal amplification (TSA) uses HRP-conjugated antibodies and hydrogen peroxide to catalyze the binding of fluorophores to proteins in the tissue sample being stained.
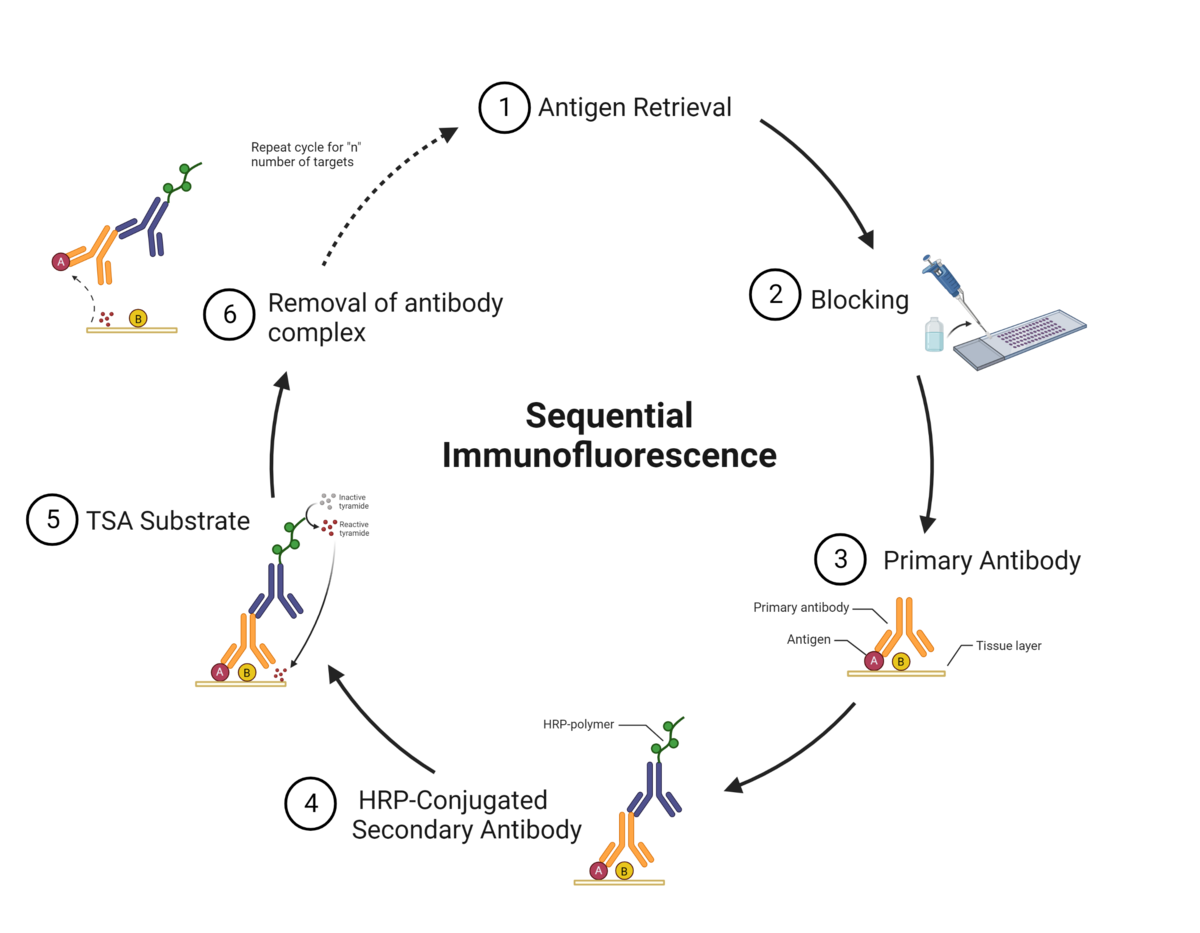
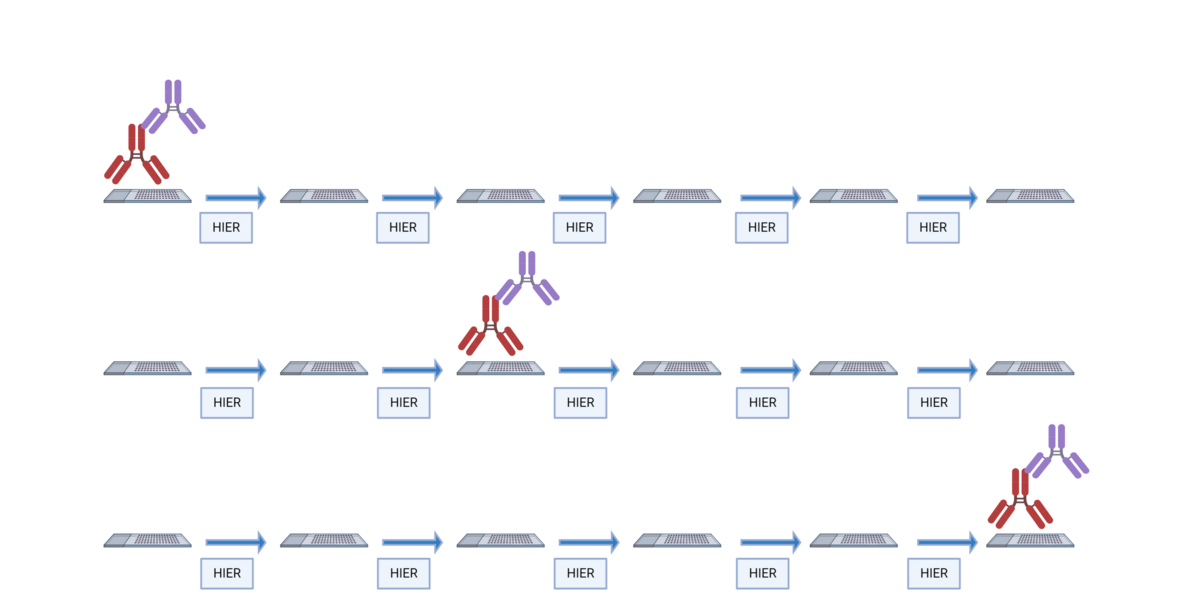
The TSA method of multiplex immunofluorescence is achieved by utilizing a basic protocol in which the primary and secondary antibodies corresponding to the first target of interest are deposited. These are then removed with heat-induced epitope retrieval (HIER), and the staining process is repeated for "n" number of targets.
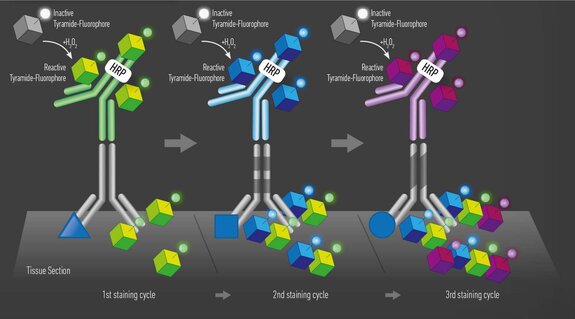
The order in which targets are applied to the tissue plays a major role in antibody activity and background intensity. Adding each antibody in the optimal position is key to ensuring a successful experiment.
There are several reasons why the order of antibodies impacts the outcome of the staining:
- Repeated heating and cooling: Multiple HIER steps can alter the epitope/antibody interaction.
- Tyramide blocking: Tyramide deposited by previous staining steps can block the primary antibody in subsequent steps.
- Tyramide trapping or caging: Deposition of tyramide may prevent the primary antibody from being removed, causing detection at subsequent staining steps.
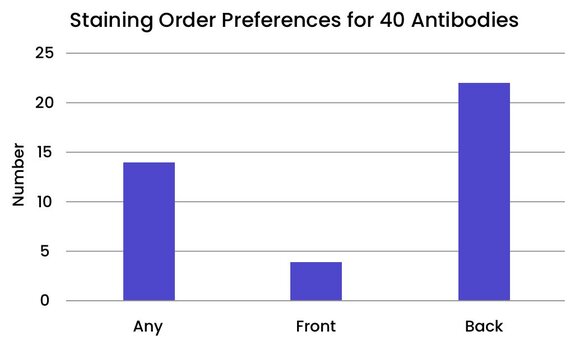
Some antibodies are more sensitive to their placement in the staining order than others. We evaluated 40 antibodies from the Bethyl catalog to determine their optimal placement in the staining order.
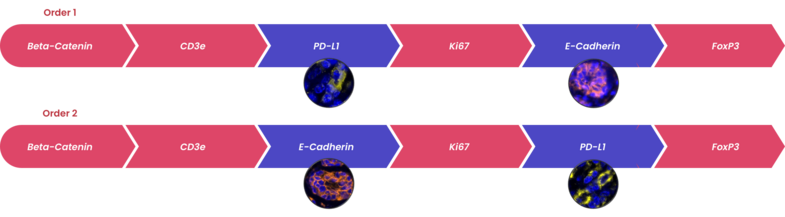
TSA is useful for amplifying the signal from a low-abundance protein in the tissue. The technique also produces high-resolution images because the fluorescent marker is enzymatically bound to the protein of interest.
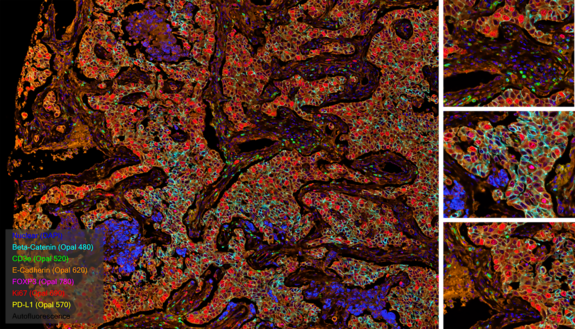
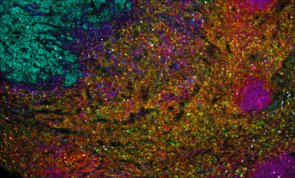
Invasive ductal carcinoma Grade I stained with 6-plex mIF.
At Fortis, we use TSA on slides produced for the Akoya PhenoImager™ HT using Opal™ reagents to generate up to 6-plex images.
Cyclic Immunofluorescence
Cyclic immunofluorescence (cyclic IF or CyCIF1 and originally referred to as MxIF2) is a high-plex imaging technique. Multiple fluorophore-conjugated antibodies are added to the slide in each round of staining. After each round of imaging, the fluorescent dyes are inactivated by chemical inactivation, photobleaching, or detergent-based stripping so that the same fluorophores can be used in subsequent rounds.2 By using the same fluorophores in multiple rounds of staining, cyclic IF minimizes the need for the use of uncommon fluorophores and extensive optimization.
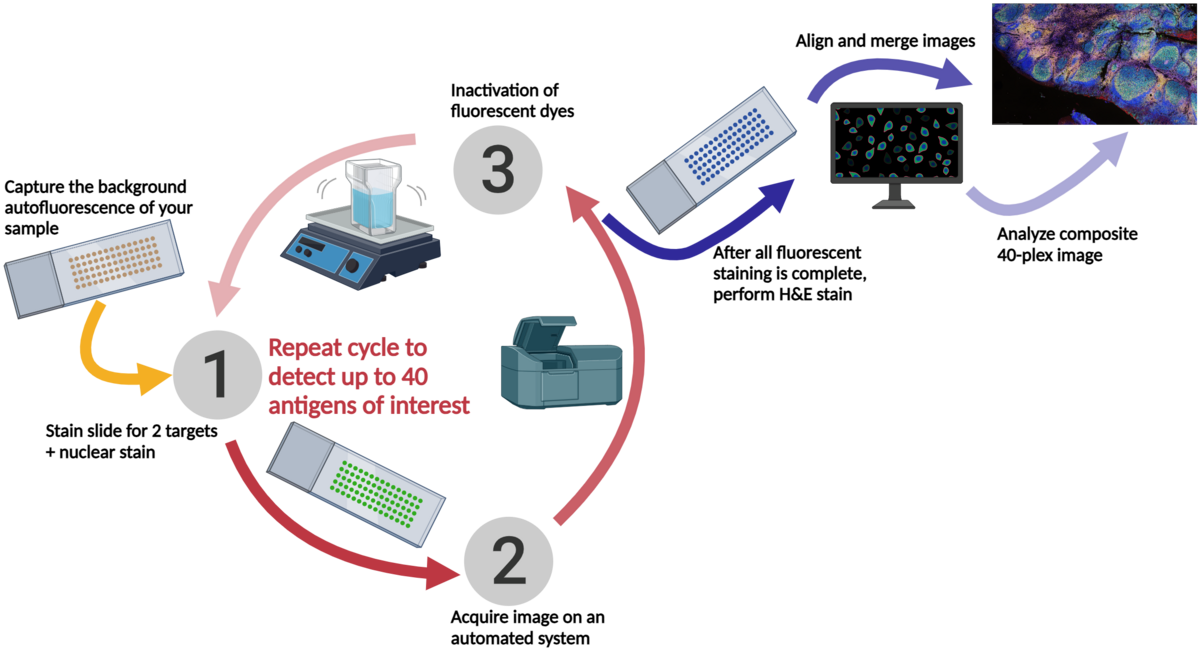
The initial publication describing cyclic IF2 demonstrated that the repeated dye inactivation did not decrease epitope or tissue integrity. DAPI or Hoechst 33342 should be included in all rounds of staining to use as a reference point for image merging and analysis.
This iterative process can be performed multiple times, allowing for the detection of multiple antigens in the same tissue sample. The images acquired after each round of staining are all merged prior to being analyzed, using nuclear staining as an alignment guide.
At Fortis, we use sequential immunofluorescence (seqIF™) on the Lunaphore COMET™. It involves successive cycles of staining-imaging-elution, without any human intervention. COMET™ uniquely preserves sample morphology and epitope integrity by efficiently removing antibodies and residues from the sample. This enables samples to be reused downstream for other applications like H&E, IHC, spatial transcriptomics, or sequencing.
References
- CyCIF.org, RRID:SCR_016267
- Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue
Fortis Life Sciences and Bethyl are registered trademarks of Fortis Life Sciences.
COMET and seqIF are registered trademarks of Lunaphore Technologies S.A.
PhenoImager, Opal, and Akoya Biosciences are registered trademarks of Akoya Biosciences, Inc.
Products & Services
Additional Resources
Multiplexing & Spatial Biology FAQs
What do I need to get started with mIF?
Can I use any primary antibody?
What types of samples do I need?
Where do I purchase tyramide signal fluorophores?
Do I have to perform any optimization steps?
Why do I observe signals during singleplex stains but not multiplex stains?
Do you have any suggestions for antigens that are localized to the same cell type within tissue?
What do I need to perform heat-induced epitope retrieval (HIER)?
How many targets can I detect in one tissue sample?