For Targets That Demand Precision Choose VHH Domains
Program success depends on selecting an antibody modality that aligns with the structural biology of the target. IgG scaffolds can be limited when recessed epitopes, constrained surfaces, conformational pockets, or multi component assemblies are involved.
×
VHH Domains Offer Solutions Conventional Antibodies Cannot
Natural single-domain architectures reach structurally complex epitopes, support modular and small format designs, integrate cleanly into multispecifics, and behave predictably in standard engineering and CMC workflows.
Evaluating VHH as a primary option can:
1
Expand the accessible epitope space
2
Improve downstream engineering options
3
Reduce the risk of avoidable attrition
Modality choice is a scientific decision that shapes everything that follows.
Why Feasibility Should Lead Antibody Discovery
VHHs are the single variable regions of camelid heavy-chain–only antibodies. They function as fully independent antigen-binding domains without a light chain, giving them a compact structure and distinctive biochemical behavior.
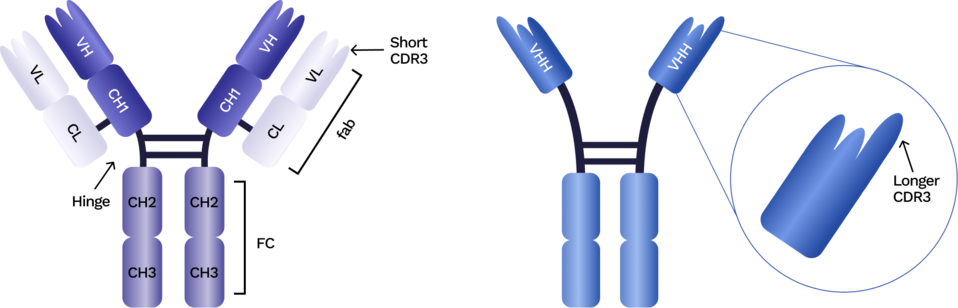
This flexibility enables:
- Small size (~14 kDa) that supports deeper tissue penetration
- High stability under heat, pH, dehydration, and buffer stress
- Extended CDR3 loops that reach recessed or cryptic epitopes
- Human-compatible frameworks similar to human VH3-23
- Monomeric format ideal for modular assembly into multispecifics
These features allow VHH domains to remain functional in demanding assay conditions and to bind structural environments that challenge conventional antibody formats, making them valuable across discovery, engineering, and translational research.
VHH Advantages for Complex Targets and Advanced Formats
- Access to cryptic, recessed, or highly structured epitopes
- Straightforward incorporation into bispecific, trispecific, and modular constructs
- Reduced aggregation due to hydrophilic frameworks
- Reliable folding and expression across multiple host systems
- Compatibility with CAR-T and CAR-NK architectures
- Strong performance in high-stress assay or buffer environments
- Clean sequence context that simplifies downstream humanization
Applications
- CAR-T and CAR-NK targeting domains
- Bispecific and trispecific antibody engineering
- Immune agonists and immune checkpoint modulators
- Live-cell binders and receptor-specific modulators
- Structural biology including cryo-EM and crystallography
- Enzyme inhibition and conformational stabilization
- High-performance diagnostics
- Bioprocess and analytical assay development
Therapeutic Areas
- Immuno-oncology
- Leukemia and lymphoma
- Myeloma
- Solid tumors
- Autoimmune disease
- Neurodegenerative disorders
- Infectious disease
- Metabolic and receptor biology
Choosing the Right VHH Source
These features allow VHH domains to remain functional in demanding assay conditions and to bind structural environments that challenge conventional antibody formats, making them valuable across discovery, engineering, and translational research.
| VHH TYPE | Immune-Derived VHH | Naïve Camelid VHH Libraries | Synthetic or Semi-Synthetic VHH |
|---|---|---|---|
| How They Are Generated | Immunization of camelids with a recombinant protein, peptide, cell, or complex antigen | Library construction from peripheral B cells of non-immunized camelids | Constructed through rational design, CDR engineering, or grafting using in silico modeling |
| Strengths |
Highest functional diversity Natural CDR3 length and structure patterns Enrichment for antigen-specific binders Strong developability and stable frameworks |
Broad baseline diversity Natural germline variation Consistent framework performance |
Controlled design parameters Good for benchmarking, scalable and consistent |
| Limitations |
Requires access to camelids Dependent on immunogen quality |
Lower initial affinity May require additional selection rounds |
Diversity constrained by design Non-natural CDR3 distributions Potential scaffold instability |
| Best For |
Difficult epitopes High-affinity binders Early therapeutic exploration Ortholog or isoform-specific targeting |
Early discovery screening Targets not amenable to immunization Teams performing internal panning |
Rapid prototyping High-throughput screens Assay development where extreme affinity is not required |
A No-Trade-Off Immune-Derived VHH Platform
Our integrated VHH discovery platform handles diverse antigen formats and yields lead-quality binders with the diversity, stability, manufacturability, and humanization compatibility you need. Experts in heavy-chain-only immune responses and complex targets guide each discovery stage.
Access High-Diversity, Immune- Derived Libraries
You start with VHH repertoires shaped by natural immune selection. We build libraries designed for rapid hit identification. Focused repertoires, including the AbNano™ Anti-T-Cell Library, reach scales of up to one billion unique sequences and enable binding to difficult or structurally complex targets.
Advance Your Program With Fast, High-Quality Binders
Accelerated workflows deliver potent binders in weeks. Your team receives live cell binders, enzyme-specific inhibitors, or affinity-validated hits to targets such as PD L1, CD3ε, and CD8αβ across a broad range of therapeutic applications.
Reduce Risk With Experienced Oversight From Idea to Lead
Your discovery path is guided by a technical staff with decades of experience in immune-response design, molecular biology, and selection strategy. Plus, our coordinated workflows deliver predictable timelines and consistent, high-quality outputs from initial concept through final lead sequence.
Fit VHH Discovery to Your Program Goals
Therapeutic, diagnostic, academic, and emerging biotech teams can pursue early hits, unique binding profiles, ortholog or isoform selectivity, and epitopes not tractable with conventional antibodies.
Explore Our VHH Library Portfolio
Looking for off-the-shelf repertoires for internal panning? Fortis offers ready-to-use AbNano libraries for rapid and independent discovery.
AbNano™
VHH Naïve Library
A natural, high-diversity repertoire built from 103 naïve camelids. Includes validated binders to PD L1, EGFR, and INSR β.
AbNano™
Anti-T-Cell VHH Library
An immune derived repertoire focused on T cell surface proteins. Nearly one billion unique sequences with validated binders to CD3ε and CD8αβ.
Explore Our Resources

Anti-T-Cell Immunization Strategy Poster
See quantitative diversity analysis, hit rates, and affinity data from the AbNano™ Anti-T-Cell Library development.

VHH Antibody Engineering eBook
Explore scaffolds, engineering strategies, and real-world therapeutic applications of VHH antibodies.

VHH Antibody Engineering White Paper
Learn features of single-domain antibodies, advantages over conventional formats, and methods of VHH library construction
Beyond mAbs - The Unrealized Potential of VHH Antibodies
Discover how VHH domains are transforming therapeutic development.

What Labs Ask Us
VHH, also referred to as single-domain antibodies, are antigen-binding fragments derived from the variable heavy domain (VHH) of heavy chain-only antibodies. These antibodies are found in camelids, such as llamas and alpacas.
Single-domain antibodies are 15kDa in molecular weight with dimensions of approximately 2.5nm in diameter and 4nm in height. Single-domain antibodies are ~10 times smaller than conventional antibodies, which are ~150kDa in molecular weight.
We provide VHH antibody discovery services and off-the-shelf VHH discovery libraries. We provide research use only deliverables, but we offer consultation on single-domain antibody sequence selection toward specific diagnostic or therapeutic objectives.