Lateral Flow Rapid Test Assay Optimization
Lateral flow assays consist of many different critical components, with each component requiring systematic optimization in order to develop a precise and accurate test. Few assays begin with the target analytical range, sensitivity, or clinical performance without issues that need to be resolved.
The optimization process typically includes choosing the appropriate antibody pair, conjugation conditions, sample pad material and treatment, conjugate pad material and treatment, nitrocellulose membrane, test line concentration, wick pad material, running buffer, cassette, and sample volume.
For quantitative assays, the standardization analyte and matrix will also have to be optimized. There is a relationship between all of these components that needs to be carefully balanced to produce an effective and functional assay. Accordingly, the development process is not linear. After each stage of optimization, the preceding stages may need to be revisited and re-optimized, resulting in an iterative and recursive process - see figure 1 below for more.

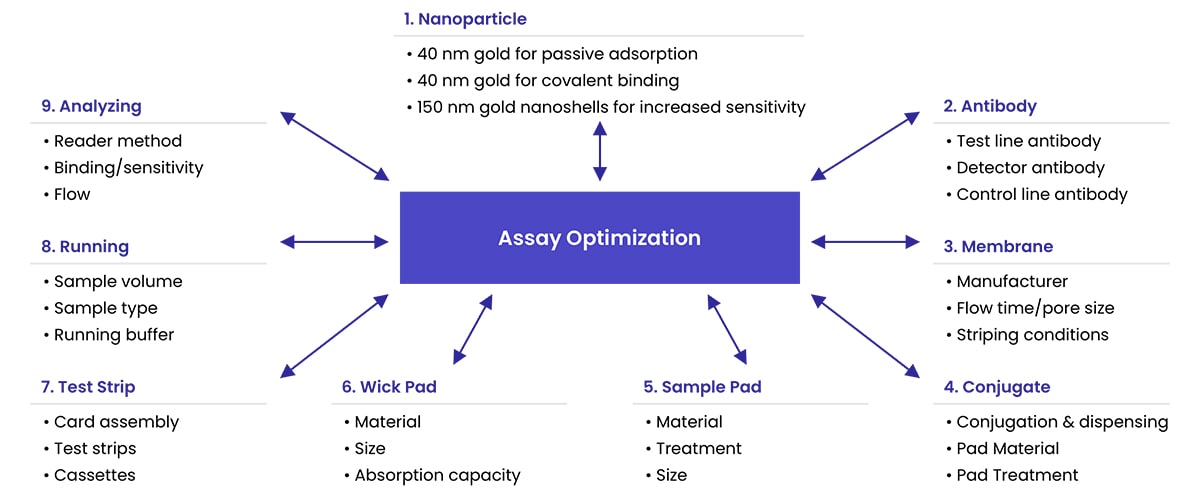
Lateral Flow Assay Optimization Tips
Optimizing the methodology of your lateral flow test will typically start with optimising individual components of the assay, highlighted in figure 1.
However, there are general optimization tips which can be investigated:
-
Sensitivity & Specificity
-
Single Variables
-
Antibody and Antigen Pairing
-
Component Optimization
-
Ongoing Optimization
-
Prioritize Optimization Goals
Tip One: Sensitivity & Specificity
When optimizing a lateral flow immunoassay, two critical metrics are the impact on signal intensity and the elimination of non-specific binding. Sensitivity and specificity, the two most essential analytical characteristics of a lateral flow test assay, are directly tied to these metrics.
Tip Two: Single Variables
It’s common to want to combine different optimization experiments to reduce testing time. However, by changing multiple variables at once, it can be difficult to interpret results, so it is recommended to make small, systematic changes in a single variable at a time during optimization.
Tip Three: Antibody and Antigen Pairing
An important decision in the design of a lateral flow assay is the pairing of the test line antibody and the antibody or antigen conjugated to the reporter particle. Each pairing between the test line antibody and conjugate is different and may require its own optimization. If you have two pairs that are comparable in performance during the initial screening, separate optimization may result in one pair having much better performance than the other. Keep in mind that a pair which works in one orientation may or may not perform the same in the opposite configuration.
Tip Four: Component Optimization
Narrow down the antibody pairs early. If you are in the fortunate position to have multiple antibody pairs that work, it is recommended to take only one or two through the component optimization.
Tip Five: Ongoing Optimization
Lateral flow assay components do not function independently, and the relationship among each of these components needs to be carefully balanced to produce an effective and functional assay. After each stage of optimization, you may need to revisit preceding stages to re-optimize. Keeping a detailed history of experiments performed will help track changes over time and inform decisions on which experiments to revisit.
Tip Six: Prioritize Optimization Goals
Prioritize your optimization goals. Early in development, typically the focus is on increasing the specific signal while decreasing the non-specific binding. Later in development, optimization can have a different focus, such as decreasing the assay run time or filtering out potential interferents.
Assay Standardization
In order to achieve the necessary accuracy required of a diagnostic assay, you will typically need to develop a method to standardize the test. This is especially true for quantitative assays.
With a standardized assay, a sample tested on your assay at different times and/or different locations will produce the same measurement for a target analyte. This reproducibility and accuracy is critical for the assay to serve its purpose in clinical or analytical applications.
The process of standardization typically begins with the analyte. Usually, the analytes used for standardization are highly purified native proteins, recombinant proteins, or other analytically detectable molecules. Whichever you choose, you need to make sure it is representative of the target analyte in the actual sample that will be tested by the end user. The standardization analyte will be used to correlate test line signal strength with the concentration of the analyte to generate a dose response curve for subsequent quantification. In some cases, the actual concentration of the standard is not known and the units are reported as arbitrary units such as “milli-international units of biological activity per liter of serum (mIU/L)”.
Regardless of the units, finding a stable, well characterized analyte standard is important for establishing a reliable calibration range. If possible, it is ideal to acquire the same antigen used to generate the antibody immune response. Commercial antibody vendors may be able to supply this information and are a good source for acquiring analytes for standardization.
Once the target analyte is selected, you will also need to optimize the matrix in which the standards are run. Standards, whether controls or calibrators, are typically prepared in the target sample matrix. This is done in order to keep the relationship between test samples and standards as close as possible. Some matrices, like urine, can have significant donor to donor differences.
In this case, you will have to optimize and test multiple sources of individual and pooled donors until you find a matrix that is representative of the target sample. In the end, you will have developed well characterized standards that can be used for each test to ensure accurate and reliable results, independent of where or when testing is performed.
Conclusion
Developing a lateral flow test is not always as simple as choosing an antigen-antibody pairing and putting the physical test components together. There are many factors at play that can impact the results from these immunoassay tests, but the optimization and standardization tips featured in this article should assist with putting together a successful R&D plan.
By clicking “Acknowledge”, you consent to our website's use of cookies to give you the most relevant experience by remembering your preferences and to analyze our website traffic.

