Upgrade Your T-Cell Marker Research With Bethyl Antibodies
Rapid progress in the field of cancer immunotherapy is a result of advances in our understanding of immune marker expression, antigen presentation, and the cell-mediated immune response involving CD3+ T-lymphocytes. T-cells are crucial to the body’s immune response to cancer, both endogenously and following therapeutic intervention. A T-cell marker panel that can identify subsets of tumor-associated T-cells allows scientists to identify and target subpopulations of T-cells playing unique roles in the immune response against a tumor.
CD4+ and CD8+ T-cells participate in the anti-tumor immune response at the site of the tumor or in the periphery, such as in tumor-draining lymph nodes. Among the T-cells localized to the tumor, some enter the tumor as naïve cells and undergo maturation within the tumor1. Others are primed in the periphery and subsequently traffic to the site of the tumor to participate in the effector or memory response against an established tumor 2. Adhesion molecules including CD313, CD434 and CD445 help regulate the migration of these T-cells.
Within the tumor microenvironment, a positive prognosis is usually conferred to patients bearing solid tumors with a high CD8+ T-cell infiltrate6, as tumor-infiltrating cytotoxic T-cells are the crucial mediator of anti-tumor immune responses. However, CD8+ T-cell activity can be regulated within the tumor microenvironment. Myeloid cells, CD4+ helper T-cells, and tumor cells can modulate the level of both maturation and activation of a CD8+ T-cell. Among many, T-cell exhaustion and anergy are major challenges to the maintenance of anti-tumor immune responses7, and can be monitored via changes to cell surface molecules.
In parallel, CD4+ T-cells can support an ongoing immune response against solid tumors through the promotion of a Th1-skewed microenvironment that primes CD8+ T-cells. Th17-polarized CD4+ T-cells, too, have recently been shown in mouse models to promote anti-tumor immune responses, possibly due to their memory-like phenotype. However, depending on both the type of cancer and the phenotype of the T-cells, CD4+ T-cells may confer either a positive or negative prognosis. While helper and memory CD4+ T-cells bolster the inflammatory response, regulatory T-cells suppress ongoing immune responses and can promote tumor progression8.
T-cell marker research is crucial to our understanding of how the immune response to a tumor is affected. Being able to categorize subsets of T-cells based on their physiologic localization and maturation state in a variety of human cancer types and corresponding mouse models will improve the ability to target crucial T-cell subsets for activation or inhibition at different stages of tumor progression. Additionally, characterizing molecules that may function as novel immune checkpoints or exhaustion markers within the context of cancer, including CD59 and CD8, can build on the successes of anti-CTLA4 and anti-PD-1 therapies, translating this field into the clinic yet again.
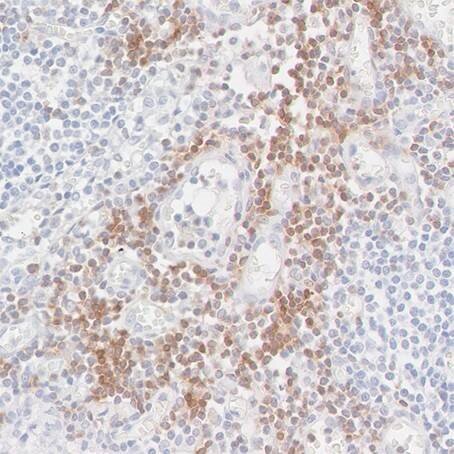
Detection of CD247/CD3Z in a FFPE section of metastatic lymph node from lung cancer origin by IHC. Antibody: Rabbit anti-CD247/CD3Z recombinant monoclonal antibody [BL-336-1B2], Cat# A700-017. Detection: DAB.
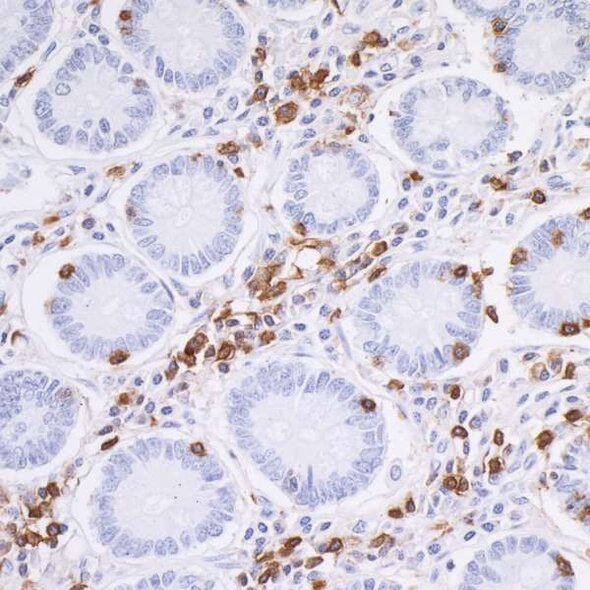
Detection of CD45 in a FFPE section of human small intestine by IHC. Antibody: Rabbit anti-CD45 recombinant monoclonal antibody [BL-178-12C7], Cat# A700-012. Detection: DAB.
References
- Sautès-Fridman C, Lawand M, Giraldo NA, Kaplon H, Germain C, Fridman WH, Dieu-Nosjean MC. 2016. Tertiary Lymphoid Structures in Cancers: Prognostic Value, Regulation, and Manipulation for Therapeutic Intervention. Front. Immunol. 7, 407.
- Slaney CY, Kershaw MH, Darcy PK. 2014. Trafficking of T Cells into Tumors. Cancer Res. 74(24). 7168-7174.
- Marelli-Berg FM, Clement M, Mauro C, Caligiuri G. 2013. An immunologist’s guide to CD31 function in T-cells. J Cell Sci 126, 2343-2352.
- Mody PD, Cannon JL, Bandukwala HS, Blaine KM, Schilling AB, Swier K, Sperling AI. 2007. Signaling through CD43 regulates CD4 T-cell trafficking. Blood. 110(8), 2974-2982.
- Baaten BJ, Li CR, Bradley LM. 2010. Multifaceted regulation of T cells by CD44. Commun Integr Biol. 3(6), 508-512.
- 6. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. 2012. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer 12, 298-306.
- Wherry EJ, Kurachi M. 2015. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol 15, 485-499.
- Kim HJ, Cantor H. 2014. CD4 T-cell Subsets and Tumor Immunity: The Helpful and the Not-so-Helpful. Cancer Immunol Res. Feb;2(2):91-8.
- Tabbekh M, Mokrani-Hammani M, Bismuth G, Mami-Chouaib F. 2013. T-cell modulatory properties of CD5 and its role in antitumor immune responses. Oncoimmunology 2(1), e22841.