Antibody Selection and Purification for Lateral Flow Rapid Tests
Fundamental to the performance of a lateral flow assay are the affinity reagents that recognize the biological target, utilized on both the particle and the test strip itself. Antibodies are a common choice that are sensitive and selective for the specific detection of very low concentrations of analyte. While aptamers and various other affinity reagents are also options, antibodies are the primary affinity reagent used for lateral flow rapid tests.
Antibodies are Y-shaped proteins that are produced by an animal’s adaptive immune system to recognize specific antigens such as viruses, bacteria or other chemicals. Typical antibodies have a molecular weight of approximately 150 kDa which corresponds to a size of 13.7 nm (width), 8.4 nm (height), (Horng 2008). One of the primary challenges of building a highly reproducible lateral flow assay arises from the variability between antibodies. The unique properties of each individual antibody make them ideal for building a highly specific diagnostic, but can also make the development of a lateral flow assay difficult and time-consuming. Each antibody requires different optimization parameters to fully utilize their binding properties.
One decision to make early in the development process is to decide whether to use polyclonal or monoclonal antibodies. Polyclonal antibodies are prepared from immunized animals. They consist of complex mixtures of different antibodies produced by many different B cell clones in the animal. Every host species and even every individual host will have a different immune response, so there is inherent inconsistency from animal to animal, and even variation from one bleed of a single animal to the next. In contrast, monoclonal antibodies are homogeneous antibody preparations produced in the laboratory. They are specific to a single antigen binding site, and produced by a single B cell clone. As a result, polyclonals may have higher recognition ability due to multiple types of antibodies targeting the antigen, whereas monoclonals are more consistent since they target exactly one epitope. Another advantage is that the cell clones used to produce monoclonal antibodies can be regenerated in the lab indefinitely, while an animal host used to generate polyclonal antibodies will eventually die. These design choices are described below in further detail.
Selection of the optimal antibodies is a critical aspect of lateral flow assay design. The ultimate performance of the lateral flow assay depends on the affinities of the antibodies used to bind an analyte in the sample. For a sandwich assay, two antibodies that can simultaneously bind to the target analyte with high sensitivity and specificity are selected. In the case of a competitive assay, the reagents will consist of one antibody and an analyte-protein competition reagent.
Commercial Antibodies vs. Custom Development
Antibodies for lateral flow development can be sourced commercially or they can be made on a custom basis against a particular antigen using a contract service. The number of commercially available antibodies for a specific analyte varies greatly and unpredictably and is dependent on the current medical and commercial market drives for detecting a particular analyte. When selecting commercially available antibodies, as many antibodies as possible should be acquired to perform initial screening, and determine which are most functionally effective either in antibody pair (sandwich assay format) or antibody-antigen pairs (competitive assay format).
Custom antibody development is a great option if there are no antibodies commercially available, or if it is important to own the antibody clone that works best for the assay, without having to license the clone from a commercial supplier upon moving to manufacture. To a certain extent, the choice of using commercially available or generating custom antibodies is a business decision since custom development can be slow and expensive but allows greater certainty of supply and potentially lower costs when manufacturing at scale.
One often overlooked component of choosing antibodies is their specificity. It is very important that the antibodies selected for optimization do not react with other components of the sample, or bind to analytes that have a similar structure to the analyte of interest. This can lead to false positive results when moving to clinical testing, so it is best to pre-emptively avoid this possibility with early cross-reactivity testing.
Testing in Lateral Flow vs. Enzyme Linked Immunosorbent Assays (ELISA)
Whether you are relying on commercially available antibodies or custom development, it is best to screen and select the antibodies by testing in the lateral flow format. Antibodies perform differently in lateral flow than in other formats such as an ELISA where the kinetics and sample matrix issues can be less important. In lateral flow, the antibody must remain active after being conjugated to the nanoparticles, retain its structural integrity when dried, and be instantly reactive upon rehydration of the sample. Traditional screening methods, such as ELISA or Western Blot may not meet these requirements as these assays usually have wash steps along with much longer incubation times, compared to lateral flow where the binding to the test line must occur in just a few seconds. Given the very short contact time, the kinetics of the antibody binding in lateral flow has a greater impact on the test result. ELISA may be useful in ruling out antibodies when beginning with a large number of clones, and incubation times can be decreased to more closely mimic lateral flow. However, we recommend screening antibody pairs in a lateral flow format as early as possible in the development process.
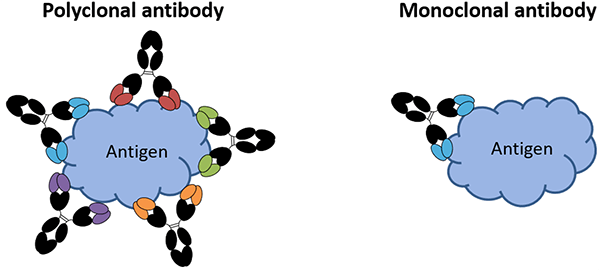
Monoclonal vs. Polyclonal Antibodies
As mentioned above, monoclonal antibodies are antibodies that have been grown from a single cloned hybridoma. Consequently, they are structurally identical and recognize only a single epitope on an antigen. By contrast, polyclonal antibodies are a heterogenous mixture of antibodies that potentially recognize multiple epitopes on an antigen.
Figure 1 – Example diagram of a polyclonal vs. a monoclonal antibody
There are advantages and disadvantages to using monoclonal or polyclonal antibodies in a lateral flow test. Monoclonal antibodies are often selected for conjugation to the nanoparticle because there is less variability between conjugations, they often have high specificity to the antigen, and they are less likely to cross-link the nanoparticles in the presence of the sample, which can happen when several conjugated particles bind to multiple different epitopes of an analyte. When this occurs, the particles can form aggregates that can cause the particles to get trapped in the membrane and not reach the test line. Polyclonal antibodies are often used at the test line due to the high affinity and ability to recognize multiple epitopes on the antigen, increasing the chances of capturing as much of the analyte as possible as it flows past. However, because of the nature of the production of polyclonal antibodies, using them in an assay is generally considered a manufacturing risk. In some cases, two monoclonal antibodies can be used in a sandwich assay if they recognize different epitopes on the antigen, and if both antibodies can bind to the analyte without interfering with each other (steric hindrance). Two polyclonal antibodies can also be used in a sandwich assay, but this requires empirical testing to demonstrate that the detector particles will not aggregate or cross-link in the presence of analyte. The advantages and disadvantages of polycolonal and monoclonal antibodies are summarized in the following table.
| Polyclonal | Monoclonal | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
Antibody Purification
For conjugation of antibodies to nanoparticles, it is critical that the antibody is in the correct buffer. For both passive adsorption and covalent conjugation, the buffer needs to be free of additional stabilizing proteins such as BSA. For passive adsorption, salt preservatives must also be avoided, and the pH of the buffer should be optimized to improve the conjugation efficiency. For covalent conjugation, the antibody buffer needs to be free of other additives that contain amines (e.g. sodium azide or tris buffer). The amines on these molecules will compete with the amines in the antibody for conjugation sites on the particles, reducing efficiency and reproducibility. For best results, the antibody for conjugation should be purified and adjusted to a concentration of at least 1 mg/mL in a low ionic strength buffer. We recommend starting with 10 mM potassium phosphate, or any buffer similar to that provided by the vendor. If necessary, this buffer formulation can be modified, including the type and molarity of salts and other components as well as the pH. Antibodies can be purified and transferred into an amine-free buffer using spin columns or dialysis tubing with the appropriate molecular weight cut-off. Spin columns with a 10 kD cutoff are provided by Fortis Life Sciences as part of the BioReady Conjugation Kit, and are appropriate for purification of most antibodies.
To purify antibodies from additional stabilizing proteins, an affinity column such as a protein A or G column is required. Since most protocols for separation with affinity columns use tris as a buffer, subsequent purification is still necessary to remove free amines after the antibody is recovered. Therefore, whenever possible, obtain antibodies without any additional stabilizing proteins. Additionally, glycerol, a common component in antibody preservation, makes the antibody solution more difficult to work with and should also be avoided.
After protein purification, the concentration of antibody should be verified to ensure that the correct amount of antibody is being conjugated to the nanoparticle. There are several ways to measure protein concentration, including absorbance at 280 nm, a BCA assay, or a Bradford assay. At Fortis Life Sciences, we measure A280 as it is simple and accurate provided all other proteins have been removed from solution. For a detailed protocol on how to purify antibodies click here.
Antibody Selection
When selecting antibodies for use in lateral flow, it is important to empirically test the available pairs to determine which pair works best. Antibodies can be conjugated via passive adsorption methods, or through different routes of covalent binding.
At Fortis Life Sciences, we’ve developed detailed protocols for our BioReady products to help accommodate your preference for passive or covalent binding to the nanoparticle surface. If you're interested in performing passive conjugation, please check out our Citrate BioReady nanoparticles and passive conjugation video tutorial.
For researchers who are new to covalent binding methods, Fortis Life Sciences offers lateral flow conjugation starter kits that contains all of the reagents and instructions needed to successfully bind antibodies to gold nanoparticles. These kits utilize EDC/Sulfo-NHS chemistry to link carboxyl groups on the particle surface to amine residues on the antibody. Fortis Life Sciences also sells NHS-functionalized particles that can be conjugated to antibodies in a single step, though we do recommend transitioning to the carboxyl-functionalized particles and performing the EDC/Sulfo-NHS activation in-house once a functional antibody pair has been selected to facilitate a more scalable process. To learn more, take a look at our covalent conjugation video tutorial or follow the link to read more about covalent conjugation here.
To empirically test antibodies for a sandwich assay, a matrix of lateral flow test strips is created where each antibody is immobilized separately as a capture reagent (immobilized at the test line) and each antibody is used as the detector (conjugated to the nanoparticle). The full table must be tested, rather than only only half, because an antibody pair that works in one detector-capture orientation may fail when the detector and capture antibodies are swapped. For example, if screening 5 antibodies, a sample screening matrix is outlined in the table below.
| Antibody #1 | Antibody #2 | Antibody #3 | Antibody #4 | Antibody #5 | |
| Antibody #1 | 1 × 2 | 1 × 3 | 1 × 4 | 1 × 5 | |
| Antibody #2 | 2 × 1 | 2 × 3 | 2 × 4 | 2 × 5 | |
| Antibody #3 | 3 × 1 | 3 × 2 | 3 × 4 | 3 × 5 | |
| Antibody #4 | 4 × 1 | 4 × 2 | 4 × 3 | 4 × 5 | |
| Antibody #5 | 5 × 1 | 5 × 2 | 5 × 3 | 5 × 4 |
After preparing the conjugates, we analyze the stability of the conjugates via UV-Visible spectroscopy and their performance on the lateral flow test strip. For each combination, or pair, we run two strips, one with a negative sample, and one with a positive sample. At this stage in development, it is preferable to use buffer with the analyte added as the sample, rather than a patient sample, if possible. This is a "cleaner" system that will yield easier to interpret results, though further optimization will be required when transitioning to the true sample matrix. After the strips have run, they are analyzed for the presence of any non-specific binding at the test line for negative samples, and the intensity of the test line for the positive samples. The antibody pair that results in the lowest non-specific binding with the highest positive binding is selected for optimization. In cases where there are several pairs that lead to promising results, other factors are important in the selection. For example, it is important to perform cross-reactivity experiments to ensure that the selected antibodies are not recognizing other analytes that will be present in a clinical sample. Since so much work goes into the subsequent optimization of the lateral flow test with the selected antibodies, potential cross-reactivity should be evaluated as early in development as possible. If a commercial antibody source is used in the down-selected pair, the antibody supplier should be approached to determine if there are any limitations on the use of the particle for commercial applications, if there is a decrease in the price for bulk purchases, and if there are any other commercial sources of the antibody available so you are not limited to a single source. Another consideration is the running conditions of the test strip. Ultimately, the assay will require total release of the conjugate without aggregation of the particles, as well as full clearance of the membrane. These factors are antibody-dependent and may require further independent optimization.
Control Line Antibodies
In both sandwich and competitive assay formats, it is important to incorporate a second line on the membrane that functions as an internal quality control. This control line will be visible regardless of the presence of analyte, and shows the end user that the assay is functional and that the results are valid. The control line antibody should be a secondary antibody specific to the species of the conjugated antibody. For example, the monoclonal antibody conjugated to the nanoparticle is often derived from a mouse host. In this system, a secondary antibody that is specific for mouse antibodies (eg. Goat anti-Mouse) will bind the conjugated antibody even in the absence of analyte and result in a visual readout. If the conjugated antibody is from a different species than mouse, the secondary antibody used at the control line needs to be specific for that species.
Read next – learn more about reporter nanoparticle selection.
By clicking “Acknowledge”, you consent to our website's use of cookies to give you the most relevant experience by remembering your preferences and to analyze our website traffic.